New Treatment for OCD Sufferers
The FDA has approved Brainsway TMS therapy for the treatment of OCD (Obsessive Compulsive Disorder). For so called “treatment resistant” OCD patients who don’t derive
The FDA has approved Brainsway TMS therapy for the treatment of OCD (Obsessive Compulsive Disorder). For so called “treatment resistant” OCD patients who don’t derive

This video show the biological changes that occur in the brain- to cause depression. It also shows how TMS therapy reverses these biological changes.z

Many of the most common antidepressants carry a risk of unpleasant side effects. These include: Nausea, Dizziness, Constipation, Anxiety or restlessness, Blurred vision, Weight gain,
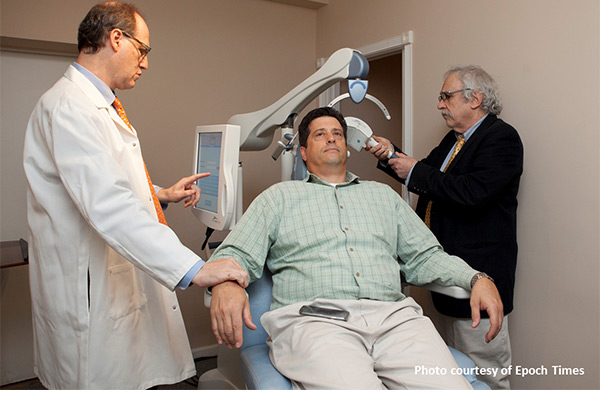
TMS Therapy Depression is an illness that affects more than 14 million adults in the United States. Since each individual is unique, medication will not

Activities When You Need a Mood Boost If you are having a bad day or just feeling stressed out take some time out of your

Depression is a common disorder that affects millions of people all over the world. There are treatments out there that can help, however they are

Those who suffer from depression understand how harrowing it is to live with on a daily basis. While therapy and medication can alleviate your symptoms,
Fibromyalgia can be a painful and debilitating illness, often presenting with co-morbid depression / other mood disorders. Limited efficacy and significant side-effects from pharmacotherapy and

Winter Blues can be sub-clinical depression or may be a Major Depression Disorder known as Seasonal Affective Disorder or SAD. Winter Blues and SAD can

Dr. Alan Manevitz is interviewed by Dr. Max Gomez on the recent study of successfully treating depression and insomnia at the same time, doubling success